

The SGTB is governed by the following committees and groups, as described below.
• Steering Committee
• Tissue Utilization Committee
• Bioinformatics and Data Management Group
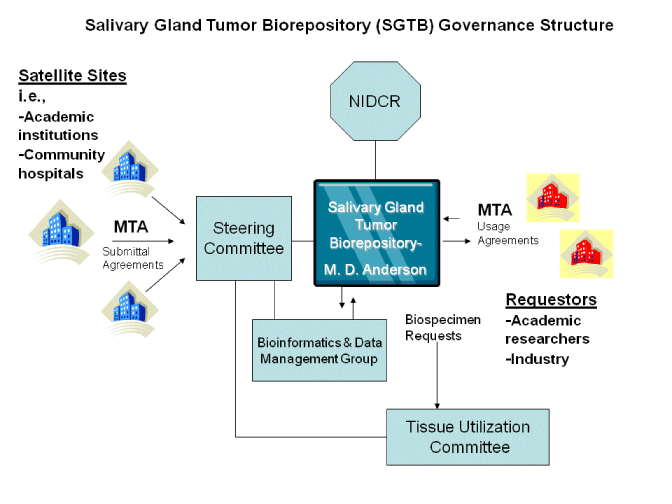
The Steering Committee (SC) is the main organizational, decision-making structure of the SGTB. The SC establishes objectives, policies, and plans of action for the SGTB. This includes the development of management Access policies/procedures; Standard Operating Procedures (SOPs) for specimen collection, storage, and distribution and guidelines for day-to-day operations including specimen acquisition, quality control, retrieval and shipping. The steering committee will also oversee the activities of both the Tissue Utilization Committee and the Bioinformatics and Data Management Group.
Current members of the SC include:
• Dr. Lillian Shum (NIDCR)
• Dr. Jim Vaught (NCI – Office of Biorepositories and Biospecimen Research)
• Dr. Adel K. El-Naggar (MDACC - PI) / Dr. Diana Bell (acting Co-PI)
• Rotating members from participating satellite centers
- Dr. Henry Frierson (University of Virginia)
- Dr. Raja R. Seethala (University of Pittsburg)
• Dr. Roger Aamodt- non voting member, setting best practices
The SC meets quarterly and as needed by conference calls.
The Tissue Utilization Committee (TUC) receives and reviews applications for specimen use, plans for tissue accrual and disbursement through MDACC, and ensures appropriate availability and prioritization of requested materials. It also ensures the economic utilization of biospecimen by coordinating related and/or overlapping research requests.
Members of the TUC include:
• An External Scientific Review (ESR) group consists of 3 academic scientists with expertise in biologically and epidemiologically-based cancer studies;
- Dr. Fredrick Kaye (University of Florida)
- Dr. Ricardo Lloyd (Mayo Clinic)
- Dr. Goran Stenman (University of Gothenburg)
• Dr. Lillian Shum (NIDCR)
• Dr. Diana Bell (acting Co-PI)
• Dr. Roger Aamodt
• Mr. Jeffrey Kaufman (Adenoid Cystic Carcinoma Research Foundation)
• Ad hoc reviewers, if additional expertise is needed.
A satellite representative serves on the TUC for a two year rotation.
The TUC receives applications for review on the last business day of each month.
The Bioinformatics and Data Management Group (BDMG) supports all aspects of biospecimen resource including research participant enrollment and consent; biospecimen collection, processing, storage and dissemination; QA/QC; collection of research participant data; data security; reporting functions; clinical annotations to the biospecimens.
BDMG consists of
• Dr. Lillian Shum (NIDCR)
• Dr. El-Naggar (MDACC)
• Dr. J. Jack Lee (MDACC)
• Waree Rinsuronkawong (MDACC)
• A representative from each satellite center
The BDMG meets quarterly via conference call.