Multc Lean
Multc Lean is for the design of single arm clinical trials monitoring
response (efficacy) and toxicity (safety). These are typically phase II
clinical trials.
Requirements
- Windows 7
(other Windows versions may be compatible but this has
not been tested)
- Administrative permissions may be required to install Multc Lean
Desktop depending on the chosen installation location.
-
The following packages will be installed if they are not present:
- Microsoft .Net 4.0 Framework
- Microsoft Visual C++ 2010 x86 Redistributable 10.0.40219
- Microsoft Windows Installer 3.1
-
To view the
Multc Lean user's guide
and
statistical tutorial,
a PDF file viewer (not included with the software) such as Adobe Reader
(available for free
here) must be installed.
-
To view the
example protocol memo,
Microsoft Word 2010 or later, or another program which can display
Microsoft Word 2010 files (not included with the software) must be
installed.
-
To follow the "Send feed back via email" link in the
Help -> About Multc Lean Desktop ... window, an email client such as
Microsoft Outlook (not included with the software) must be installed.
What's New in Version 2.1
-
The standard treatment event rate may be modeled as a constant rate
instead of a random variable with a Beta distribution
-
Updated documentation, including a new
example protocol memo
- Significant improvements to the user interface
- Minor bug fixes
-
Occasionally sends usage statistics and crash reports to our biostatistics software
support team to improve your experience using the software.
What's New in Version 2.0
-
Improved display of stopping boundaries (details
here)
- Updated documentation
Description
Multc Lean implements a special case of the single-arm safety monitoring
method of Thall, Simon, and Estey [1]. The method of
Thall, Simon, and Estey is a general family of Bayesian designs for monitoring
phase II trials. The method of Thall, Simon, and Estey can be used to monitor
any number of outcomes.
The Multc Lean software only monitors two outcomes. This is a
limitation of (or simplification provided by) the software implementation, not
the statistical method.
The Multc Lean software also allows a modification to the method, whereby
the standard treatment rate may be modeled by a fixed constant rate, instead of
a random variable with a Beta distribution.
Features
- A Windows 7 user interface
- Extensive documentation
- The ability to simulate trial duration
- Support for continuous monitoring or monitoring in cohorts
- Support for a minimum number of patients to treat before evaluating the
stopping rules
- Support for modeling standard treatment rates as constant rates instead
of random variables with a Beta distribution
- A “lean” implementation which is very easy to use
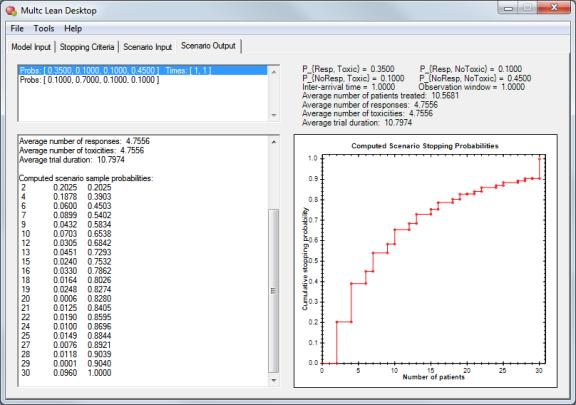
Why Bayesian Trial Designs?
An advantage of Bayesian methods is that they allow arbitrary sample sizes.
One starts with an uninformative probability distribution on the parameters of
interest (such as the probabilities of toxicity and response) and ends with a
more informative posterior distribution after the trial. There is no
all-or-nothing threshold where n patients are too few but n+1
patients are plenty. The uncertainty in the posterior parameter estimates
decreases continuously as the number of patients in the trial increases. Small
studies are not disallowed; they simply have more posterior uncertainty than
larger studies.
Documentation
An extensive statistical tutorial
is provided with the software. This tutorial covers the statistical basis of
the method, has guidelines for how to use the method and includes exercises and
solutions. See also the
Multc Lean user's guide
included with the software.
An example protocol memo
is provided with the software. This is intended to help those, often statisticians, who are designing
clinical trials using Multc Lean Desktop to communicate the design to colleagues.
As such it is an example only, and would need to be heavily edited to match any new design. By no
means do we mean to imply that this is the best or only way to communicate a design, but we hope it
may be helpful.
Conducting a trial designed by this method does not require software since
the stopping conditions can be tabulated before the trial begins. Here is a
document commenting on the
logistics of running a Multc trial
for the benefit of the person responsible for monitoring the stopping rules.
Alternative Software
Multc Lean was designed to have a “lean” implementation
which is very easy to use and retains only the most commonly used features of
the
original Multc99
program. Multc99 allows one to monitor any practical number of events. Multc99
is a command-line menu-driven text-based program with limited documentation but
more flexibility than Multc Lean Desktop.
Multc Lean Desktop version 2.1 now allows you to design trials with the same
stopping criteria as Multc99's “Phase IIA with Binary Outcome”
designs, which are modifications of the original designs proposed by Thall,
Simon, and Estey [1]. These designs, which model the
standard treatment event rate as a fixed constant instead of a random variable
with a Beta distribution, are some of the most frequently chosen designs
supported by Multc99. Multc Lean also allows extensions to Multc99's
“Phase IIA with Binary Outcome” designs, such as the ability to use
a cohort size greater than one, to obtain operating characteristics when
monitoring both response and toxicity events, and to simulate the average trial
duration.
Credits
Hoang Nguyen developed the original MultcLean program using Microsoft
C#. John
Venier added the enhancements present in versions 2.0 and 2.1.
John Cook and Hoang
Nguyen implemented the numerical algorithms using Microsoft Visual C++.
References
[1]
Peter Thall,
Richard Simon, and
Elihu Estey in “Bayesian
sequential monitoring designs for single-arm clinical trials with multiple
outcomes”,
Statistics in Medicine,
vol 14, 357-379 (1995)
 Biostatistics Software --- Desktop / Cloud
Biostatistics Software --- Desktop / Cloud
