BMA-CRM Simulator
The BMA-CRM Simulator is an easy-to-use implementation of both the BMA-CRM and DA-CRM
dose finding methods.
Bayesian Model Averaging with Continual Reassessment
Method by Guosheng Yin and Ying Yuan.
Bayesian Data Augmentation Dose Finding with Continual
Reassessment Method and Delayed Toxicity by Suyu Liu, Guosheng Yin
and Ying Yuan.
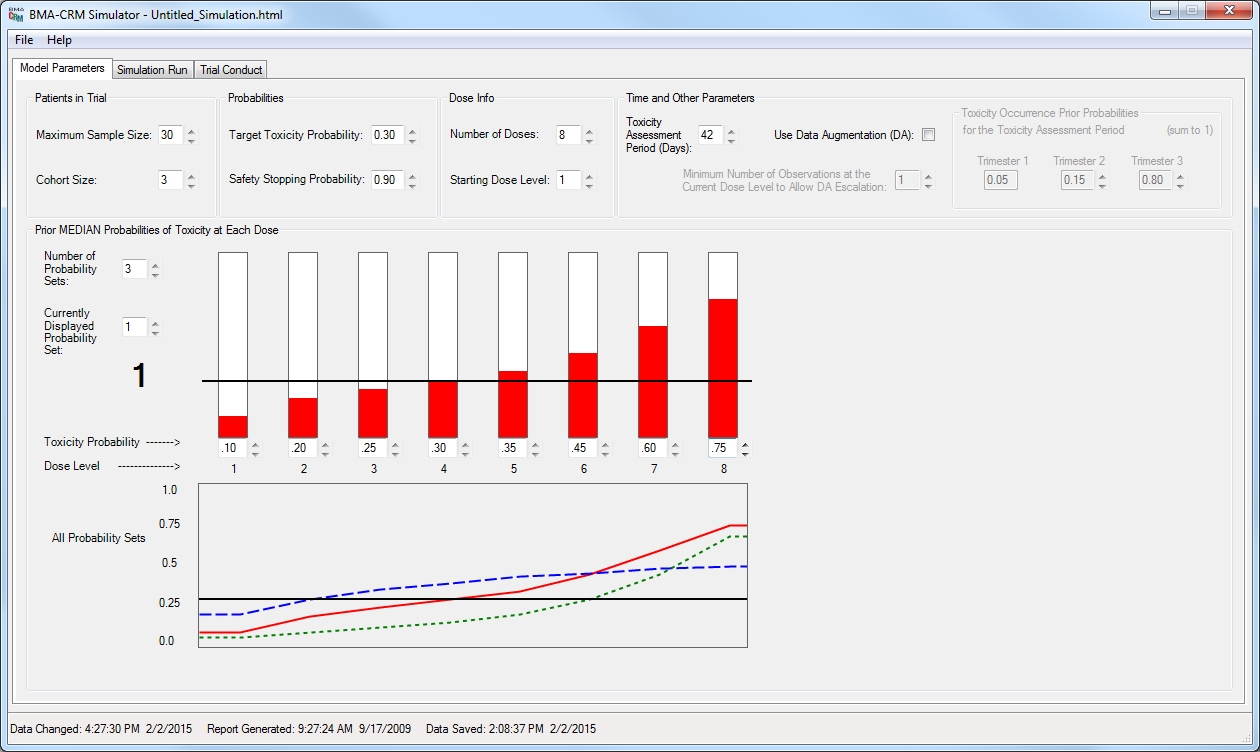
See also the BMA Method
Description, the DA
Method Description and the
User's Guide available here and also included with the software.
NOTE: See the “What’s New” sections below.
Note that this version (2.2.4) of BMA-CRM Simulator comes with the User's Guide
for version 2.2.1. We are updating the User's Guide, but didn't want to
disappoint by delaying the availability of this widely requested current version
of BMA-CRM Simulator. In the meantime, we have added many tooltips and messages
to help you operate the software.
The software was developed by John Aven, Richard Herrick, Clift Norris, John Venier
and Lin Zhang. The software tools were: Microsoft Visual Studio 2013; C# for
the user interface, and C++ for the calculation engine. (February, 2018)
System Requirements
- Windows 7 SP1
- Microsoft .NET Framework version 4.5.2 (x86 and x64)
- Microsoft Visual C++ 2013 Runtime Libraries (x86)
- Windows Installer 4.5
- Minimum Screen Resolution 1280x769
If any required software component is absent from your system, the installation
process will install it.
What's New in V2.2.4
- An issue with the installer has been fixed.
- There are other improvements.
What was New in V2.2.3
- Menus are modernized; a minor bug in the “Recent Files” system is fixed.
- The .NET Framework used has been upgraded to 4.5.2.
- There are other improvements.
What was New in V2.2.2
-
The Trial Conduct Tab is Operational! The Trial Conduct tab has been completely
rewritten to improve it and to accommodate
trials which use Data Augmentation. Now you can delete patients, get a trial conduct
decision without committing to treating a
patient, view the state of the trial at any point in its history, and more. Each
trial decision now comes with an extensive
description explaining the details of the decision and how it was obtained.
-
Scenario Grid: Scenarios can now be given meaningful names and can be reordered.
-
MTD Determination: The rules for determining the MTD have been changed to
better match users' expectations and actual practice.
Note that this change should not affect well-designed trials whose maximum sample
size is reasonably large (see below).
The MTD is now required to have had at least three patients treated on it. If the
dose level closest to the target probability
of toxicity without skipping untried dose levels has had fewer than three patients
treated on it, the highest dose level lower than
this dose level which has had at least three patients treated on it is determined
to be the MTD. If no such dose level exists, the MTD
is determined to be the dose level closest to the target probability of toxicity
without skipping untried dose levels, but you are
warned that the determination of the MTD has a high degree of uncertainty because
there were too few patients treated at that dose
level.
-
Maximum Sample Size: We now recommend that the maximum sample size be at
least three times the number of dose levels. If a
lower maximum sample size is specified, we warn you and include a warning in the
simulation output.
-
Dose Level Escalation: For increased safety and to better match users'
expectations and actual practice, we now limit dose
level escalation in some circumstances. We now do not allow escalation away from
a dose level for which the current raw proportion of
toxicities is above the target probability of toxicity. That is to say, if the
proportion of toxicities among all currently fully observed
patient outcomes at a dose level is above the target probability of toxicity, we
do not allow escalation away from that dose level. If
escalation would otherwise be recommended but this rule applies, it is still recommended
to treat a patient, but the dose level on which the
patient should be treated is lower than it would otherwise be. Note that in the
rare circumstance that this rule needs to be applied to a
dose level at which no patient outcomes have been fully observed, it is assumed
that the dose level does not have a raw proportion of
toxicities above the target probability of toxicity. This is because this rare circumstance
should only arise with trial designs which
assume for other reasons that escalating away from such a dose level is acceptable.
-
Power User Features: Some features have been added for use by experts investigating
the Data Augmentation calculations. These
features are not available by default.
- Many improvements and bug fixes.
What was New in V2.2.1
-
Trial Design:
-
Data Augmentation: If desired, the trial design will use Data Augmentation
(DA) when patient
data are missing. This allows treatment decisions and trial safety decisions to
be made without
waiting for missing data to become known, greatly reducing the expected trial duration.
When DA is
chosen, you are limited to one set of prior probabilities of toxicity for the dose
levels.
More information about using Data Augmentation in CRM can be found in
DA Method Description.
When DA is chosen, you must specify additional trial design parameters specifically
for DA.
-
Toxicity Assessment Period: You now must specify the toxicity assessment
period. Of course
this needs to be specified for DA, but it is also now used in simulation with or
without DA so that
we may estimate expected trial duration.
-
Simulation:
-
Accrual Rate: You must specify the accrual rate when simulating, so that
expected trial duration may
be estimated. The accrual rate may also affect the operating characteristics when
DA is chosen.
-
Proportion of Toxicities Observed in Second Half of Assessment Period: This,
along with the probability
of toxicity, parameterizes the Weibull distribution now used to simulate time to
toxicity at each dose level.
-
Multithreading: The simulations are multithreaded on machines with more than
two cores, greatly reducing
the time required to perform them.
-
Trial Duration: Expected trial duration (total trial time) is estimated.
- Many other improvements.
What was New in V2.1.2
-
IMPORTANT: The inputs for the sets of prior probability of toxicity at each
dose
level are now user-specified as prior MEDIANS. (Previous versions expected
them
to be prior means, and calculated the corresponding prior medians.) So what
you input for these values has changed
since it is a different statistic compared
to versions older than v2.1. For the dose-toxicity model
πj(α) = pjexp(α),
the inputs for the prior probabilities of toxicity at each dose level are now used
directly as the pj values, which are the prior medians. See this
technical report
for an explanation of the prior median, and note that the
BMA Method Description
describes the old method of using the inputs as prior means, but is otherwise correct.
-
NOTE: Simulation files created with older versions will not open in v2.1.
If you
wish to use an old trial design you will need to re-enter your design, bearing in
mind
that the sets of prior probabilities of toxicity at each dose level are now prior
medians. If you use the same input values with v2.1 as you used with an older
version and re-simulate, you may obtain different results.
- Various updates and bug fixes.
- The program occasionally sends usage statistics and crash reports to our biostatistics
software support team to improve your experience using the software.
References
-
Guosheng Yin, and
Ying Yuan
(2009). Bayesian Model Averaging Continual Reassessment Method in Phase I Clinical
Trials.
Journal of American Statistical Association, 104, 954-968.
-
Suyu Liu,
Guosheng Yin, and
Ying Yuan
(2013). Bayesian Data Augmentation Dose Finding with Continual Reassessment Method
and Delayed
Toxicity. The Annals of Applied Statistics, Vol 7, No. 4, 2138-2156
 Biostatistics Software --- Desktop / Cloud
Biostatistics Software --- Desktop / Cloud
