Dose Schedule Finder
Design and conduct Phase I
clinical trials that simultaneously optimizes dose and schedule
This software is for designing and conducting Phase I clinical trials that
simultaneously optimizes both dose and schedule. The
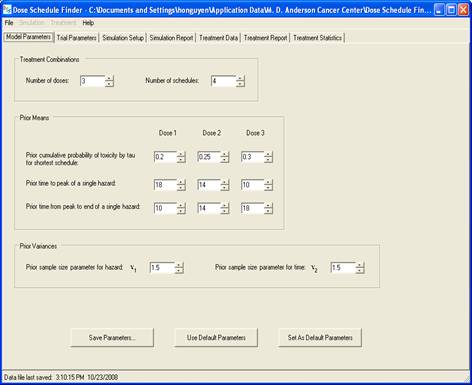
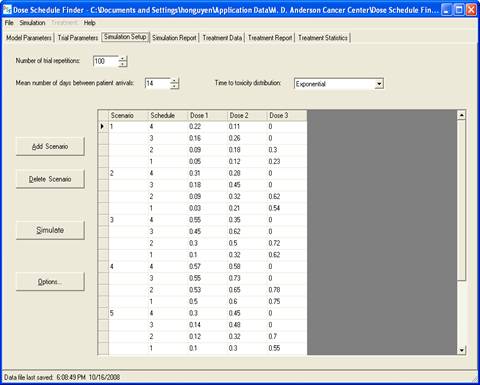
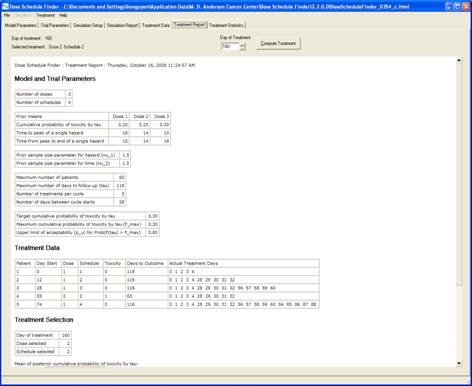
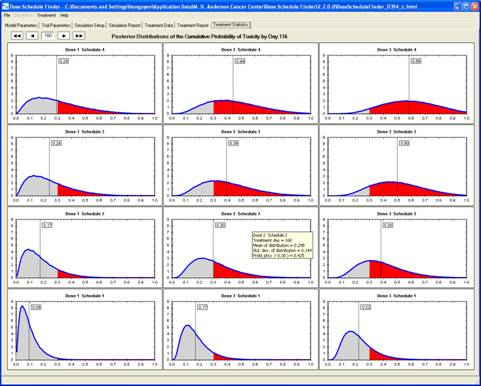
goal is to determine a maximum-tolerated dose and schedule (MTDS) in terms of
the overall risk of toxicity. The method is Bayesian
adaptive and uses time-to-toxicity as the outcome.
The software is based on the paper
Simultaneously
optimizing dose and schedule of a new cytotoxic agent, Thomas M Braun, Peter F Thall,
Hoang Nguyen, and Marcos de Lima, Clinical Trials 2007; 4: 113–124.
Hoang
Nguyen developed the numerical algorithms using Visual C++, and the user
interface using C#
and the Microsoft .NET framework version 2.0
 Biostatistics Software --- Desktop / Cloud
Biostatistics Software --- Desktop / Cloud



