Bayesian Optimal Interval (BOIN) Design Desktop Program
The BOIN Design Program provides a novel platform for designing various phase I
clinical trials, including
single-agent
and drug-combination trials, as well as trials with late-onset toxicity.
The program allows users to design trials, run simulations, and generate trial
protocols in multiple languages (English and Chinese).
Description
The software (i.e., a Windows desktop program called “BOIN”) implements
the BOIN designs [1,2] for single-agent trials,
single-agent trials with late-onset toxicity [3],
drug-combination trials seeking a single MTD [4]
or the MTD contour [5].
As a model-assisted design, the BOIN design combines the advantages of algorithm-based
designs and the advantages of model-based designs.
It can be implemented in a simple way similar to the traditional algorithm-based 3+3
design, but yields excellent performance comparable to the more complicated model-based
designs, such as the continual reassessment method (CRM) [6,
7]. The BOIN design is motivated by the top priority and concern of
clinicians, which is to effectively treat patients and minimize the chance of exposing
them to subtherapeutic or overly toxic doses.
System Requirements
- Windows 10 (may work on earlier versions of Windows as well, although this is not supported)
- Microsoft .NET Framework version 4.7.2
-
Minimum screen resolution 1318x860
Note: You can still use the program with a
smaller screen resolution, but it may require the use of scroll bars. If you have your display set
to use a text size of “Medium - 125%” or “Larger - 150%” you may need a
greater screen resolution to use the program without scroll bars.
If any required software component is absent from your system, the installation process will install it.
Features
- Self-contained Windows program
- Very easy to use
- Handles single-agent and drug-combination phase I trials
- Accommodates late-onset toxicity/fast accrual to accelerate phase I trials
- Automatically generates a protocol document template in both English and now ??!
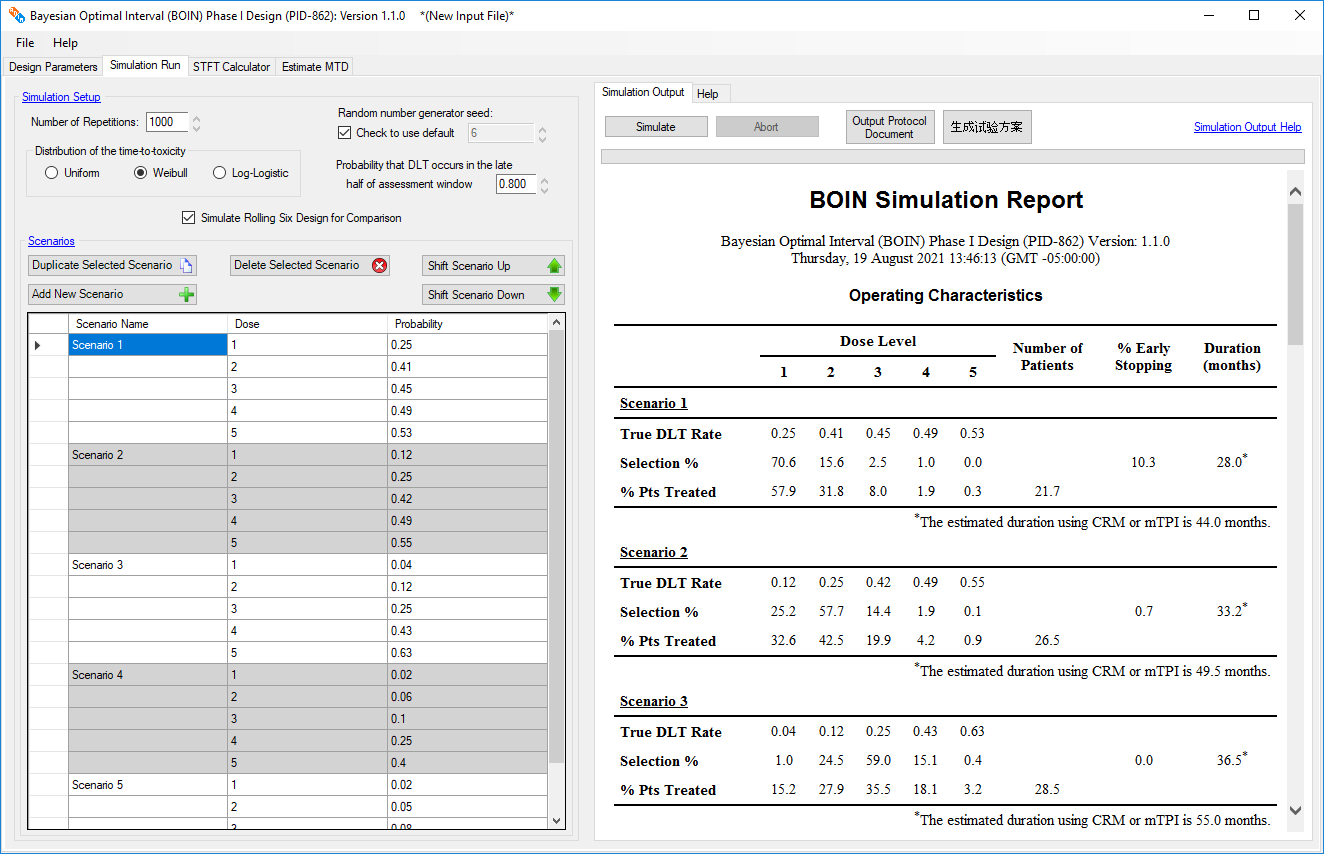
- Enables the user to generate operating characteristics for protocol preparation
-
Optionally generates operating characteristics for 3+3 and Rolling 6 trial designs for comparison
to the single-agent BOIN and TITE-BOIN designs respectively
-
A Trial Conduct tab for conducting drug-combination trials and estimating the
single MTD or MTD contour from trial data
Why use the BOIN Design?
-
Simple to implement Similar to the 3+3 design, the dose
escalation/de-escalation rule is prespecified and can be tabulated. During the trial conduct, clinicians
can simply count the number of patients who experience toxicity and compare the observed toxicity rate
with the prespecified dose escalation/de-escalation boundaries to determine dose assignment until the
trial is completed. For drug-combination trials, the dose escalation/de-escalation rule is the same but
the overall conduct of the trial is more complicated which is why we provide a trial conduct tab for
those trials. Nevertheless each decision to escalate the dose, de-escalate the dose, or remain at the
same dose is easy to understand, explain, and predict.
-
Superior performance Both theoretically and numerically, it has been shown
that the BOIN design has desirable statistical properties and superior operating
characteristics [6 , 7].
-
Highly Ethical The design optimizes the dose assignment for each patient in
the sense that it minimizes the chance of assigning a patient to either a subtherapeutic dose or an overly
toxic dose. This is consistent with the clinician’s point of view for conducting clinical trials, i.e.,
maximizing the patient’s treatment benefit.
-
Accommodates both single-agent and drug-combination trials The BOIN design
can be used to design both single-agent and drug-combination phase I trials. The resulting designs are easy
to implement.
-
Accommodates late-onset toxicity and fast accrual The time-to-event
BOIN (TITE-BOIN) design allows real-time dose assignment decisions for new patients while some enrolled
patients’ toxicity data are still pending, thereby significantly accelerating phase I trials.
-
Solid statistical justifications The design optimizes a sensible
statistical criterion. It has a desirable finite-sample property (i.e., is coherent) and a desirable
large-sample property (i.e., converges to the target dose). See the references for more details.
Documentation
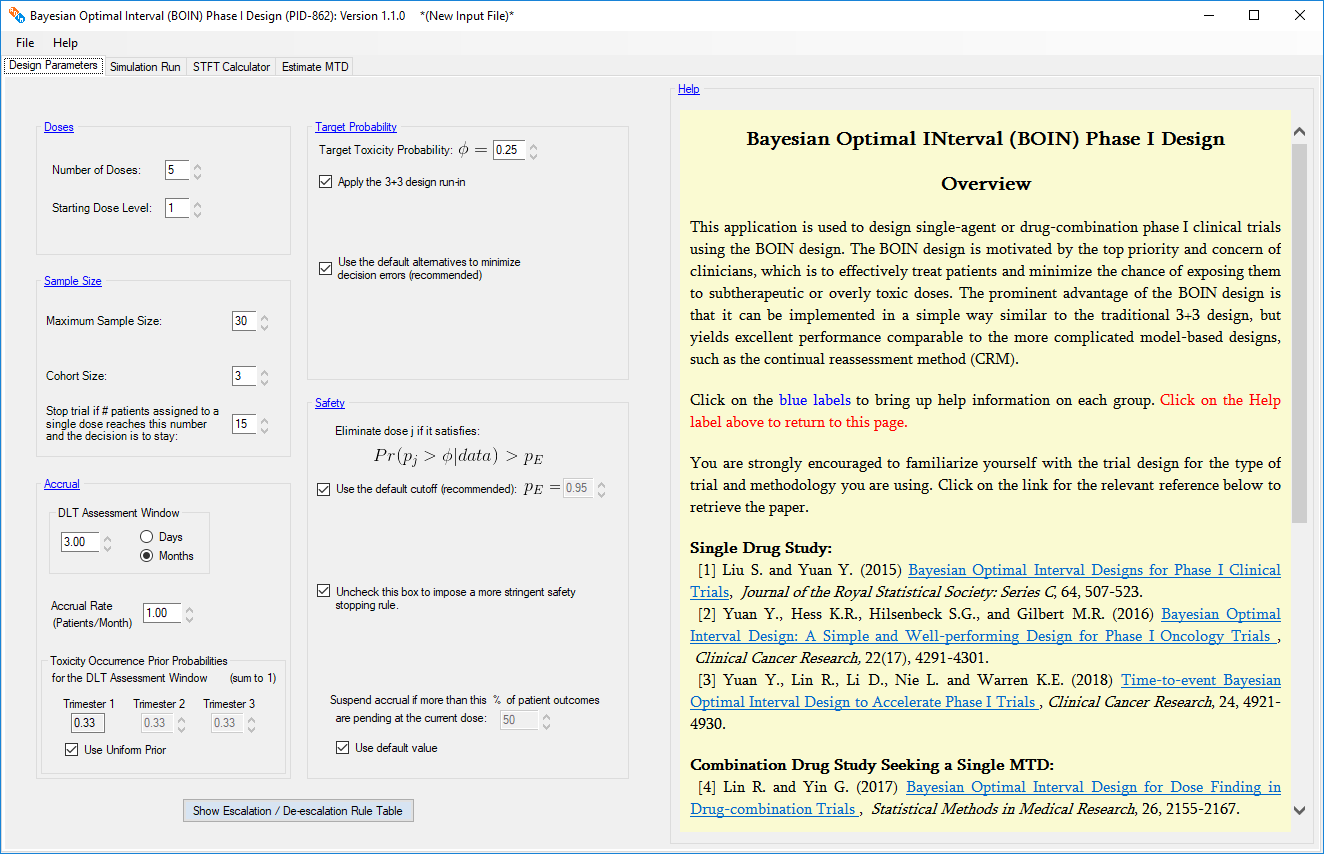
Extensive context-specific help is provided in the program itself for all aspects of the trial design and how
to use the program. The program produces simulation output which can be incorporated in other documents and
also can automatically generate a protocol document template in either Word or HTML format. An extensive
statistical tutorial, written for the
R package BOIN,
is available separately. This tutorial covers the statistical basis of the method, and has guidelines for how
to use the method.
What’s New in v1.1.0?
Added an 'Estimate MTD/MTD Contour' tab page for the BOIN COMB designs. Added a (W)STFT [(weighted)
standardized total follow-up time] calculator for even easier TITE-BOIN trial conduct. Added the capability
of launching multiple simulators of different design types, simultaneously. Various other improvements.
Credits
Richard C. Herrick, Clift Norris, John Venier, and Ying Yuan wrote this program.
Ying Yuan, Suyu Liu, Liangcai Zhang, Ruitao Lin, and Heng Zhou originally implemented the numerical
algorithms using R. Yanhong Zhou contributed to the R code.
References
Single Drug Study:
[1] Liu S. and Yuan Y. (2015)
Bayesian Optimal Interval Designs for Phase I Clinical Trials
,
Journal of the Royal Statistical Society: Series C, 64, 507-523.
[2] Yuan Y., Hess K.R., Hilsenbeck S.G. and Gilbert M.R. (2016)
Bayesian Optimal Interval Design: A Simple and Well-performing Design for Phase I Oncology Trials
,
Clinical Cancer Research, 22(17), 4291-4301.
[3] Yuan Y., Lin R., Li D., Nie L. and Warren K.E. (2018)
Time-to-event Bayesian Optimal Interval Design to Accelerate Phase I Trials
,
Clinical Cancer Research, 24, 4921-4930.
Combination Drug Study Seeking a Single MTD:
[4] Lin R. and Yin G. (2017)
Bayesian Optimal Interval Design for Dose Finding in Drug-combination Trials
,
Statistical Methods in Medical Research, 26, 2155-2167.
Combination Drug Study Seeking an MTD Contour:
[5] Zhang L. and Yuan Y. (2016)
A practical Bayesian design to identify the maximum tolerated dose contour
for drug combination trials
,
Statistics in Medicine, 35, 4924-4936.
Comparison of BOIN to Other Designs:
[6] Zhou H., Murray T., Pan H. and Yuan Y. (2018)
Comparative review of novel model-assisted designs for phase I clinical trials
, Statistics in Medicine, 37, 2208-2222.
[7] Zhou H., Yuan Y. and Nie L. (2018)
Accuracy, safety, and reliability of novel phase I trial designs
,
Clinical Cancer Research, 24(18), 4357-4364.
 Biostatistics Software --- Desktop / Cloud
Biostatistics Software --- Desktop / Cloud


