TTEDesigner
TTEDesigner: Designing one-arm time-to-event safety monitoring trials
This software is for designing single-arm safety
monitoring trials with time-to-event outcomes and a single arm. Time to event is modeled as
exponential, with mean distributed as inverse gamma. The trial stops if and
when the posterior probability is sufficiently large that the experimental
treatment is worse than a historical standard. The event being monitored is
often something bad, like disease progression, in which case you want to
maximize time to event. But the event can also be something good, like
engraftment, in which case you want to minimize the time to event. This simple
model is surprisingly robust, as demonstrated in [1].
Most of the parameters in the design have clear
interpretations and rules of thumb for filling them in. You can get suggestions
for filling in parameters by clicking on links in the software. But there's one
cutoff parameter that isn't obvious how to set. Typically people run a dozen
simulations, tweaking this parameter until they get the operating
characteristics they want. TTEDesigner seeks to eliminate that process.
Instead of entering cutoff parameter values, you enter the operating
characteristics you want and let the software solve for the parameter
that best meets those characteristics.
As with everything else in statistics, it's usually not
possible to satisfy everything at once. Everyone wants tiny trials with small
type I and type II error. This software will let you satisfy two out of three
criteria:
- Sample size
- Stopping percentage under bad scenario
- Stopping percentage under good scenario
So for a given sample size, it will come up with a
cutoff parameter that will exactly satisfy (2) and another parameter that will
exactly satisfy (3). It also gives three compromise designs for values in between these
extremes for a total of five designs. Select one of these five designs, and it
will give you a table of stopping boundaries like that in
TTEConduct.
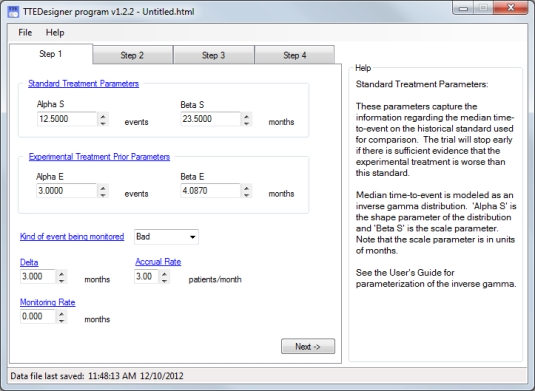
See the TTEDesigner
user's manual for more details.
Clift Norris developed the user interface for TTEDesigner using
C#. Leiko H. Wooten
and John Cook
developed the statistical calculations using Visual C++.
What's new in v1.2.2?
- IMPORTANT: DO NOT USE v1.2.1. A bug introduced in v1.2.1 led to bad simulation results. This is fixed in v1.2.2.
- Improved text.
References
-
Peter F. Thall,
Leiko H. Wooten, and
Nizar M. Tannir.
Monitoring Event Times in Early Phase Clinical Trials: Some Practical Issues,
Clinical Trials 2, 467-478 (2005).
 Biostatistics Software --- Desktop / Cloud
Biostatistics Software --- Desktop / Cloud
